| Posted on
Water dissolves a variety of minerals as it comes into contact with jewels, soil, and other geological conformations. The dissolved minerals contribute to the overall composition of water, impacting its taste, quality, and implicit health impacts. Common minerals dissolved in water include

Calcium( Ca) and Magnesium( Mg) :
These minerals contribute to water hardness. While essential for mortal health, inordinate hardness can lead to scale conformation in pipes and appliances.
Sodium( Na) and Potassium( K):
These are common ingredients, with sodium contributing to saltness. High sodium situations can affect water taste and impact individualities on sodium-confined diets.
Chloride( Cl) and Sulfate( SO4):
These ions are frequently set up in natural waters and can impact taste. Elevated situations may indicate impurity or geological sources.
Bicarbonate( HCO3) and Carbonate( CO3) :
These contribute to alkalinity, affecting the pH of water. Alkaline water can have a bitter taste and impact the erosion of pipes.
Iron( Fe) and Manganese( Mn):
This essence can be naturally present in water, leading to abrasion and affecting taste. High attention may have health counteraccusations.
Fluoride( F):
While essential for dental health in applicable attention, inordinate fluoride can lead to dental and cadaverous fluorosis.
Nitrate( NO3) and Phosphate( PO4):
These nutrients are essential for factory growth but can lead to water pollution, especially in agrarian areas.
Understanding the mineral composition of water is pivotal for water quality assessment, ensuring safe
consumption, and addressing implicit environmental and health enterprises. Water treatment processes may be employed to alleviate inordinate mineral attention and enhance overall water quality.
0
0 Comment
| Posted on
Salts form when rocks wear down. They join with rain, streams, or salt lakes. Salts break apart into ions. Ions have a charge. Charged salts mix with water. Hard salts make hard water. Hard salts form scale buildup. Scale clogs pipes. It wrecks hot water tanks and gear. Hard salts are lime, chalk, and gypsum. Some salts leach into wells and taps. Well, tap salts change the taste. A few key salts make most hard water. Calcium salts are the main culprits. Lime salts like calcium carbonate form scale. Chalk salts add haze and scale. Gypsum salts are calcium sulfate. It adds murk and scum. Magnesium salts give a bitter taste. Iron salts turn taps and laundry red. Iron oxides stain red rust hues. Iron salts may add metal tang. Salts of copper or zinc tint green and blue. These sour taste. High loads of salt pit taps.
.jpg)
Few salts boost health. A dash of salts add vital salts and trace salts. Small traces of salts like zinc, iron, and iodine are key. But hard water is harsh. Too many harsh salts soap suds and cause soap scum. It may need a saltwater softener. Streams and rain flush salts downslope to big sea ponds. Flows bear salts and salts from worn rock and soil. As runoff moves along streams, salty load shifts. Fast rapids drop some salts, yet flows take on fresh rock bite. Flows slow in floodplains, hold more salts, add gypsum, iron rust, and clay grit. Where rivers pour forth into the sea, surf belts clash with fresh flows. Mixed fresh and salt loads create briny brown surge zones. In these zones, many salts crash out, and muddy plumes rise. Bit by bit, the waters go with the salty flow. But some silt stays in lag zones. Over a long time, silt builds up, and fans out deltas. The bulk of salts are borne forth to the open sea. In the sea, the great salt flush stays afloat and brews sea soup.
0
0 Comment
university.nakul@gmail.com | Posted on
Water is much more than a transparent, tasteless liquid. It is often referred to as a “universal solvent” because of its ability to dissolve a variety of substances, including minerals. These minerals play a crucial role in the water we drink, making it not just a hydrating agent but also a source of essential nutrients. In this post, we’ll explore what it means for minerals to be dissolved in water, which minerals are commonly found, their health benefits, potential risks, and more.
1. What Does It Mean for Minerals to Be Dissolved in Water?
When water flows over rocks, soil, or through underground aquifers, it absorbs minerals that are naturally present in the environment. This process, known as mineral dissolution, occurs when minerals break down and mix with water molecules. As a result, minerals become part of the water’s composition, and this water is known as mineralized water.
The amount and type of minerals dissolved depend on several factors, including the geological makeup of the area, the water’s acidity (pH), and its temperature. This process can result in water that is either mineral-rich, such as natural spring water, or water with fewer dissolved minerals, like distilled or rainwater.
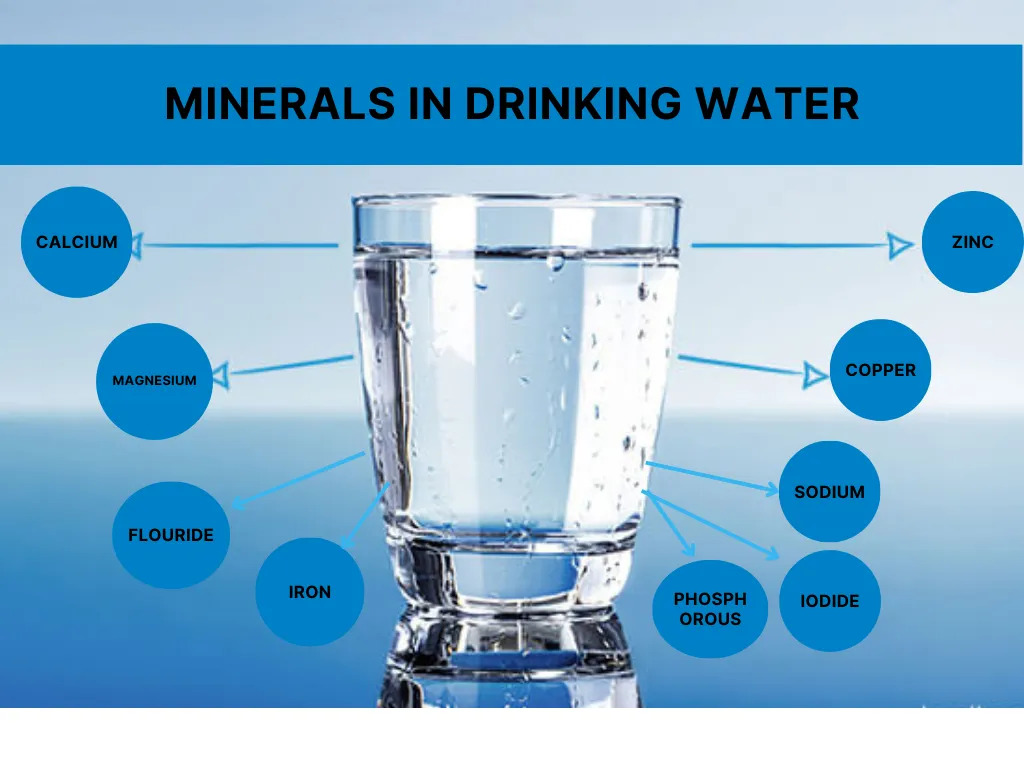
2. Common Minerals Found in Water
Several key minerals commonly dissolve in water, contributing to its taste, quality, and health benefits. These minerals include:
- Calcium: Vital for bone health and plays a role in muscle function.
- Magnesium: Supports cardiovascular health and muscle function.
- Sodium: Helps regulate blood pressure and fluid balance in the body.
- Potassium: A critical electrolyte that helps maintain fluid balance and nerve function.
- Chloride: Works with sodium to maintain the body's fluid balance.
- Bicarbonate: Helps regulate the pH of the body and contributes to digestion.
- Sulfate: Supports digestive processes and metabolism.
These minerals are naturally present in both surface and groundwater sources, making them an integral part of what we drink daily.
3. Health Benefits of Minerals in Water
Minerals dissolved in water provide more than just hydration—they offer numerous health benefits. Here's how some of these minerals contribute to overall well-being:
-
Calcium: Known for its role in building and maintaining strong bones, calcium is an essential mineral for skeletal health. Adequate calcium levels help prevent bone-related conditions like osteoporosis.
-
Magnesium: This mineral supports cardiovascular function, helping to regulate heart rhythms and prevent issues like hypertension and heart disease. Magnesium also aids in muscle relaxation and nerve function.
-
Electrolytes (Sodium, Potassium): These minerals help maintain the body's fluid balance, ensuring that cells receive the proper nutrients. Sodium and potassium are particularly important for maintaining blood pressure and supporting muscle and nerve function.
-
Bicarbonates and Sulfates: Bicarbonates help neutralize acids in the body, supporting digestion and maintaining a balanced pH. Sulfates, while less discussed, play a role in digestive processes and help metabolize proteins and fats.
Drinking mineral-rich water can be a natural way to ensure your body gets these essential nutrients, but it's important to strike a balance.
4. Water Hardness and Its Mineral Content
One of the most noticeable effects of dissolved minerals in water is water hardness. The hardness of water is determined by the concentration of calcium and magnesium, and it can affect household appliances, personal care, and even the taste of your drinking water.
-
Hard Water: Water that has a high concentration of calcium and magnesium is considered "hard." It can leave mineral deposits on dishes and appliances and may cause dry skin and hair.
-
Soft Water: Water with lower levels of these minerals is called "soft." While it doesn't have the same issues as hard water, some people find that it lacks the desirable taste that minerals can provide.
Hard water isn't harmful to health, but its high mineral content can create inconveniences in daily life, such as scaling in pipes, reduced effectiveness of soaps, and potential irritation of the skin.
5. How Are Minerals Tested in Water?
To ensure the quality of drinking water, testing for mineral content is essential, particularly in areas where natural mineral levels are high. Water testing can provide insight into the concentration of various minerals and help determine whether additional water treatment is necessary.
-
Lab-Based Tests: These are typically the most accurate and involve sending water samples to certified laboratories that test for a wide range of minerals, as well as other contaminants.
-
Home Water Testing Kits: Many people opt for home water testing kits, which can give quick results about the basic mineral content and water hardness. However, these kits may not be as comprehensive as lab tests.
-
pH Levels: The pH of water also plays a role in determining its mineral content, as more acidic water tends to dissolve more minerals from rocks and soil.
Testing for minerals ensures that water meets regulatory standards and helps prevent potential health issues from excess mineral intake.
6. Minerals in Bottled Water vs. Tap Water
There is an ongoing debate about whether bottled water, often marketed as "mineral water," is superior to tap water in terms of mineral content and health benefits.
-
Natural vs. Artificial Mineral Content: Bottled mineral water typically comes from natural springs or underground sources, where water naturally absorbs minerals. However, some bottled waters have minerals artificially added after purification, which may not offer the same benefits.
-
Comparison: In many cases, tap water contains sufficient minerals for daily needs, especially in areas with good water quality management. On the other hand, some bottled waters boast higher mineral levels, particularly those marketed as "mineral-rich."
-
Misconceptions: Many believe bottled water is always healthier, but tap water often undergoes stricter regulations and testing than bottled water.
While both bottled and tap water can provide essential minerals, it's important to verify the source and mineral content to make informed choices.

7. Potential Risks of Excess Minerals in Water
While minerals are beneficial in appropriate amounts, excessive mineral content in water can lead to health and household issues.
-
High Calcium and Magnesium: Excessive calcium and magnesium contribute to hard water, which can lead to scale buildup in pipes, reduced appliance efficiency, and even dry skin or hair.
-
Sodium and Hypertension: Water with high sodium levels may pose risks for individuals with hypertension, as sodium contributes to elevated blood pressure.
-
Sulfates: In large amounts, sulfates can cause gastrointestinal issues, such as diarrhea or dehydration.
Balancing mineral intake through water is important, as too much of a good thing can lead to undesirable effects.
8. How Can You Remove Excess Minerals From Water?
If mineral levels in water are too high, there are several methods available to soften or purify the water:
-
Ion-Exchange Systems: These systems replace calcium and magnesium ions with sodium or potassium ions, reducing water hardness. However, they may increase sodium content, which can be problematic for some individuals.
-
Reverse Osmosis: This method forces water through a semi-permeable membrane, removing most dissolved minerals. Reverse osmosis is effective but can also strip water of beneficial minerals.
-
Water Filtration Systems: Activated carbon filters and other filtration systems can reduce mineral content, but they may not remove all types of minerals.
Each method has its pros and cons, so choosing the right system depends on your specific water quality and health needs.
9. Natural Sources of Mineralized Water: Spring Water and Groundwater
Natural sources of water, such as springs and underground aquifers, are rich in minerals due to prolonged contact with rocks and soil.
-
Spring Water: Water from springs often has high mineral content, making it a popular choice for bottled mineral water. The minerals in spring water are naturally occurring and provide a distinct taste.
-
Groundwater: Like spring water, groundwater can be rich in minerals as it flows through underground layers of rock and soil. However, groundwater quality can vary depending on the surrounding environment and human activity.
Untreated natural water may carry health risks, so it's essential to test its safety before consumption.
10. The Role of Minerals in Ecosystems and Agriculture
Minerals in water are not just essential for human health—they also play a crucial role in ecosystems and agriculture.
-
Impact on Soil: Water with the right mineral content supports soil fertility, providing essential nutrients for plant growth.
-
Plant Growth: Minerals like calcium, magnesium, and potassium are vital for the development of crops and vegetation, making them critical for agricultural sustainability.
Balancing the mineral content in water used for farming is crucial for maintaining healthy crops and soil over time.
Conclusion
Minerals dissolved in water are essential to human health, environmental balance, and even agricultural sustainability. By understanding the role of these minerals in our water supply, we can appreciate the importance of water testing, mindful consumption, and balanced mineral intake. Whether you're drinking tap water, bottled water, or water from a natural spring, knowing what's in your water ensures that you're making the best choices for your health and the environment.
0
0 Comment

